

Mixtures have variable or indefinite chemical properties. The chemical properties of pure substances are constant and definite too. Mixtures have varying physical properties. The physical properties of pure substances are definite and constant. They are classified into elements and compounds based on their compositions.īased on their composition, mixtures get divided into homogeneous and heterogeneous mixtures. The physical combination of two or more substances is a mixture where the identities of the individual components are retained while the mixture is in form of colloid, suspension or solutions. Mixtures refer to substances that contain two or more different chemical substances that are not chemically combined together. Substances that are made up of only one kind of particle and has a fixed or constant structure A mixture of oil and water, soil, and sand are heterogeneous mixtures as they don’t have a uniform composition.ĭifference Between Pure Substance and Mixture Heterogeneous Mixtures – Those mixtures which do not have a uniform composition entirely are heterogeneous mixtures. Soda water, lemonade, a mixture of salt in water are a few examples of homogeneous mixtures. Homogeneous Mixtures – Those mixtures whose composition is uniform throughout the mixture is called homogeneous mixtures. Based on their compositions, mixtures can get divided into two types – homogeneous and heterogeneous mixtures. A solution of salt and water, sugar and water, air, different gases, etc. Mixtures refer to substances that contain two or more different chemical substances that are not chemically combined. Thus the atom of only one element that composes an entire molecule is not referred to as a compound. Ionic compounds are held together by ionic bonds. Elements can be metals, non-metals, and metalloids.Ĭompounds – Compound is a pure substance that is composed of many identical molecules and molecular entities that are composed of atoms that belong to more than one element held together by a chemical bond. For instance, when you break down gold, you get gold again. Such a substance cannot be broken into two or a simpler substance by physical or chemical means. By their chemical composition, pure substances can be divided into two types – elements and compounds.Įlements – An element is a pure substance that contains a single kind of atom. Gold, silver, iron, and aluminium are pure substances, to name a few.
WHEN TWO OR MORE ELEMENTS JOIN TOGETHER CHEMICALLY FREE
The substances that contain only one type of particle and are free from any mixture, are known as pure substances. So as you know, all the substances can get divided into two categories – pure substance and mixture.
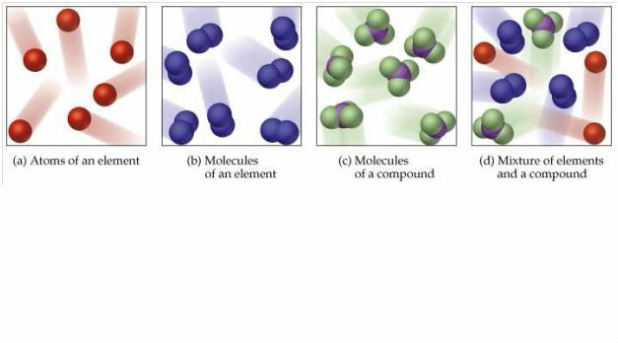
It’s important to understand what pure substance is, what a mixture is and how do they get classified further. In this article, you will learn about the difference between pure substance and mixture and fundamental concepts of the same.īefore we dive into the pure substance vs mixture comparison, you must know some essential concepts. Mixtures can get divided into two kinds, namely, homogeneous and heterogeneous mixtures.

When two or more pure substances come together, we call it a mixture. Elements and compounds are the two categories to classify pure substances further. They cannot get separated into other forms of matter. Substances that have a particular composition get called as pure substances. However, you can classify the matter in pure substances and mixtures as well. To help show this three-dimensional shape even more accurately, we can rely on space-filling models as well as ball-and-stick models.Solid, liquid, and gas are the three fundamental states of matter. We will discuss the significance of these electrons at the end of this section. The two dots above nitrogen indicate a lone pair of electrons that are not involved in any covalent bond. However, in the more detailed structural formula on the right, we have a dashed line to indicate that the rightmost hydrogen atom is sitting behind the plane of the screen, while the bold wedge indicates that the center hydrogen is sitting out in front of the plane of the screen. In the structural formula to the left, we are only seeing a two-dimensional approximation of this molecule. Keep in mind, however, that atoms and molecules, just like everything else in the universe, exist in three dimensions-they have length and width, as well as depth. From both of these structural formulas, we can see that the central nitrogen atom is connected to each hydrogen atom by a single covalent bond.


 0 kommentar(er)
0 kommentar(er)
